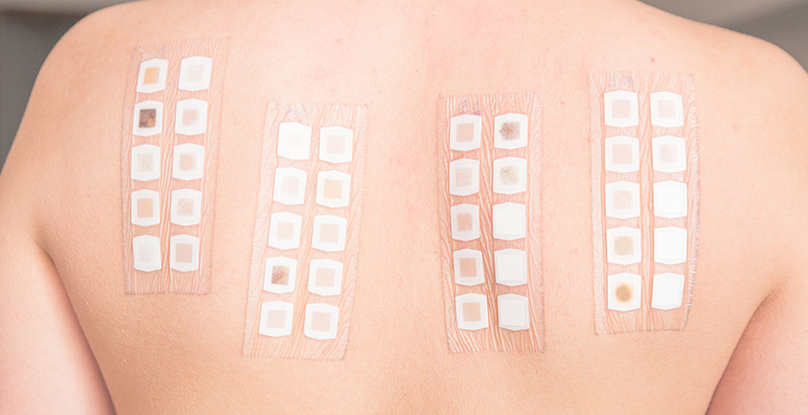
Cosmetics Registration And Record Inspection
According to "Notice on Management of the Issuance of the Recording of Domestic Non-Special Purpose Cosmetics" issued by State Food and Drug Administration (Permitted by SFDA [2011] No. 181), domestic non-special purpose cosmetics should apply to the provincial food and drug supervision and administration department for the record in the administrative area where the production enterprise is located within 2 months after the product is put on the market.
China-Norm U@Test Consumer Research Center and Shanghai Institute of Quality Inspection and Technical Research reached a strategic cooperation to jointly carry out the inspection business required for the filing of non-special purpose cosmetics. As the domestic non-special purpose cosmetics filing and inspection agency announced by the State Food and Drug Administration, Shanghai Institute of Quality Inspection and Technical Research undertakes hygiene safety inspection in the domestic non-special purpose cosmetics filing inspection.


Domestic Cosmetics for Special
purposes and Imported Cosmetics Human Safety Inspection Service
Filing Inspection
Human Safety Inspection Service

Filling of domestic non-special
purpose cosmetics Human Safety Inspection
All domestic cosmetics for special purposes and imported cosmetics must hold Cosmetics Hygiene License issued by State Food and Drug Administration (hereinafter referred to as SFDA) can be sold on the Chinese market.
To apply for Cosmetics Hygiene License, first your product needs to be experimentized by administrative permitted cosmetics inspection institution recognized by SFDA. After the completion of the inspection, the declared unit needs to prepare declaration materials according to "Cosmetics Administrative Permitted Declaration and Acceptance Provisions" (Permitted by SFDA [2009] No. 856) and send them to SFDA for assessment.
Administrative permitted inspection includes Hygiene Safety Inspection and Human Safety Inspection. As to Human Safety Inspection, U@Test Consumer Test & Research Centre in China-Norm cooperates with West China Hospital, Sichuan University, and jointly carry out Human Safety Inspection business for the registration required for domestic cosmetics for special purposes and imported cosmetics. West China Hospital, Sichuan University is a Human Safety Inspection Institution recognized by SFDA.
According to "Notice on Management of the Issuance of the Recording of Domestic Non-Special Purpose Cosmetics" issued by State Food and Drug Administration (Permitted by SFDA [2011] No. 181), domestic non-special purpose cosmetics should apply to the provincial food and drug supervision and administration department for the record in the administrative area where the production enterprise is located within 2 months after the product is put on the market.
If applying for the filing of domestic non-special purpose cosmetics, you may choose the inspection agencies designated by provincial food and drug supervision and administration departments for filing inspection. The items for filling inspection shall be determined according to those of non-special purpose cosmetics in "Specification for inspection of cosmetic administrative license" (Permitted by SFDA [2010] No. 82).
In addition to hygienic safety test, the filling of non-special purpose cosmetics also requires human safety inspection. China-Norm U@Test Consumer Research Center cooperates with West China Hospital, Sichuan University to provide daily chemical enterprises with non-special purpose cosmetic human safety inspection services. West China Hospital, Sichuan University is the human safety inspection agency for cosmetics administrative license designated by State Food and Drug Administration.
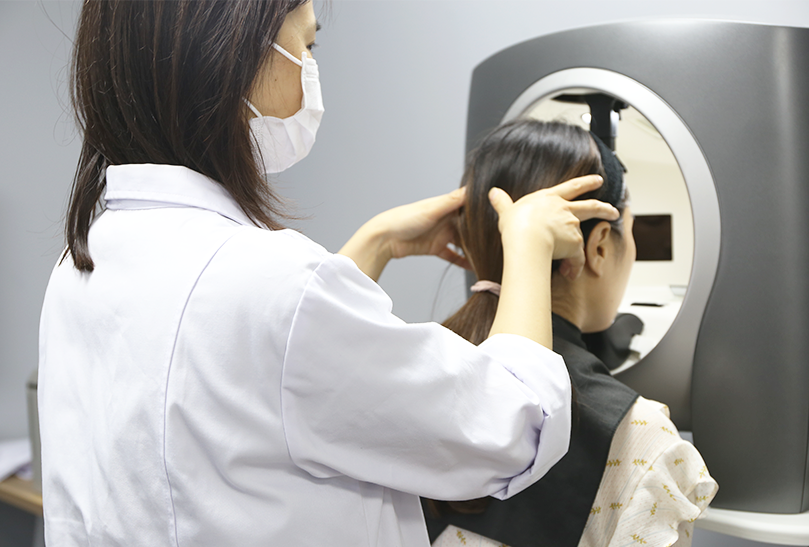
Efficacy evaluation and safety evaluation of cosmetic r&d
Our extensive experience and rigorous SOP implementation in efficacy assessment guarantee the objective and rigorous evaluation data collection based on the smooth proceeding of the evaluation project.
After the program is finalized, we will help your product efficacy test being approved by the review of Cosmetics Medical Ethics Committee. We will formulate a efficacy evaluation scheme for the product category and its corresponding efficacy you need accordingly. If you have an established efficacy evaluation program, we will provide ameliorative suggestions.
We have offered many internationally renowned cosmetics companies full-featured evaluation services, including data handling for efficacy evaluation and issuing the evaluation report after the end of the evaluation implementation. To select a full-featured evaluation services will help your business reduce costs in R & D testing.
We cooperate with many domestic Grade III Level A hospitals, administrative licensing inspection agencies, filing agencies in cosmetics safety evaluation, asserted efficacy evaluation of cosmetics for Non-special Purposes, special cosmetics efficacy evaluation and so on. We will provide authoritative efficacy evaluation report for your products in filling, registration, efficacy claims, etc.
Measurement and Evaluation Methods
Routine Measurement:
Skin Moisture Test Skin Elasticity Test Skin Moisture Loss Test Skin Melanin Test Skin Hemoglobin Test Skin pH Test Skin Color Test Skin Gloss Test Skin Surface Temperature Test Skin Friction Test Skin Grease Test Skin Wrinkle Test Scalp Grease Test Scalp Moisture Test Scalp Moisture Loss TestDigital Imaging:
Facial Image Analysis Skin Surface Texture/Wrinkle Analysis Skin Pores Analysis Skin Characteristics Analysis Makeup Characteristics AnalysisSubjective Evaluation:
Evaluation of Skin Gloss Evaluation of Skin Color Evaluation of Skin Stain Evaluation of Skin Uniformity Evaluation of Acne Severity Evaluation of Dandruff Level Evaluation of Scalp Type Evaluation of Hair Greasiness Evaluation of Scalp Dryness Evaluation of Scalp Greasiness